In 2012, Laura Duvall flew to New York to apply for a postdoctoral position with Leslie Vosshall, PhD, the Robin Chemers Neustein Professor at the Rockefeller University. Having done her PhD research on the fruit fly Drosophila melanogaster, and knowing Vosshall’s studies on the insect’s behaviors under olfactory cues, Duvall pitched her ideas about neuropeptide regulation of behaviors in Drosophila. Duvall recalls that Vosshall agreed to support her ideas, but under one condition: no fruit flies. The lab, Duvall was told, was switching completely to mosquitoes. Hearing this, Duvall was reluctant. Her projects relied on mutant animals, but there were few genetic editing tools available for mosquitoes. To build a mutant line, she anticipated, would take a year and thousands of dollars.

“But, I just realized this may be an interesting opportunity,” Duvall says. “Mosquitoes have the most extreme behaviors such as host-seeking and blood-feeding.” On the other hand, she calculated at the time, “I always have Drosophila in my back pocket if working with mosquito became a waste of time.”
The pesky insect turned out not to be a waste of time. Now, with her own lab at Columbia University since 2019, Duvall has received multiple awards for her work on mosquito behaviors, including a 2020 Beckman Young Investigator Award, a 2020 Klingenstein-Simons Fellowship Award in Neuroscience and a 2021 Pew Scholar in Biomedical Sciences. These achievements have propelled the new lab to continue the important work of mosquito behaviors and will allow Duvall to take on another challenge: to weaponize mosquitoes against themselves for vector control.
Clocking in
Duvall grew up in a very rural part of western North Carolina, in the Appalachian mountains. She left her town in high school and went to a public boarding school in Durham, North Carolina, five hours away from home, that had a science and mathematics focus. She then decided to study biochemistry at the University of Pennsylvania and found a love of science as a work-study member of a lab.
“If you had asked me what kind of major I would like to choose from my heart, it must be the art major. Unfortunately, I have zero aptitude for it,” says Duvall. “But when doing research, I discovered that I can really combine my creative impulses with my analytical and critical thinking, which I am better at, to create new knowledge.”
It was so exciting for me to think about connecting something from genes all the way to behaviors.
Laura Duvall, PhD
At the time, Duvall read a book called Time, Love, and Memory, a biography of Seymour Benzer that describes his discoveries of the genes that control the circadian rhythms, mating and memory in Drosophila. “It was so exciting for me to think about connecting something from genes all the way to behaviors,” says Duvall. Her excitement about genes and behaviors did not end with the last page of the book, and Duvall decided to come to Washington University School of Medicine for graduate school and joined the lab of Paul Taghert, PhD, Professor in the Department of Neuroscience, in 2007 to study circadian neurophysiology in the Drosophila brain. “I like the availability of tools that Drosophila has,” says Duvall, “and I especially appreciate the elegance of the Drosophila circadian system.”
The Taghert lab has been a leading lab in dissecting the mechanisms of neuromodulation in the circadian system. When Duvall arrived, one of the mysteries the lab was working to solve was about the privileged access of the neuropeptide pigment dispersing factor (PDF) to the clock cells—neurons that express core clock genes that produce the circadian rhythm. Taking advantage of the gene-manipulating tools in Drosophila, Duvall reported in 2012 that PDF receptors are coupled preferentially to the downstream pathway of the clock output, while other receptors in the same cells are not coupled in a same way. Interestingly, PDF receptors in different clock cells seem to have different partners and carry different functions. “This is how dynamic the receptor signaling can be, which happens not only in the clock network, but also as a general principle in biology,” says Duvall.

Duvall also contributed to a major finding in the lab about the daily oscillation of the sensitivity of the PDF receptor to its neuropeptide. “That was a significant finding and we were able to find later a single molecule that could lock the sensitivity in either a low or high state,” says Taghert.
Taghert is always impressed by the determination in Duvall. “Laura is able to keep steady on a daily basis,” says Taghert, “which I think makes her better at withstanding the difficulties in the laboratory work.”
Aside from learning how to do rigorous and careful research in the lab, Duvall also enjoyed the connections she had with the cohort of students at WashU. “I like going to the Clocks Club where everyone is able to talk about their work regularly with people outside of the lab. This is very important because science is about sharing the work.”
From flies to mosquitoes
Finishing up graduate school, Duvall still found herself fascinated by the question of how behaviors are regulated. So she applied to work with Vosshall, who studies animal behaviors under cues from the environment using species such as mosquitoes.
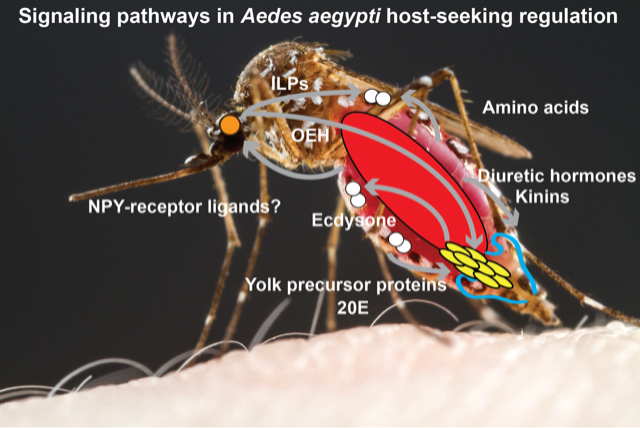
Female mosquitoes bite humans to obtain blood to feed their eggs. However, these voracious blood-suckers also have a pause in their attraction to humans for several days after blood-feeding. The mechanism behind this suppression has intrigued scientists for years. Having learned that a conserved receptor family may be involved, and that the same receptor family is a popular testing target for diet control in humans, Duvall set out to test some of these drugs on mosquitoes. “These drugs bind to the human receptor with high affinity but failed with a lack of therapeutic efficacy in human,” Duvall says. To her surprise, “it worked on mosquitoes and modified their host-seeking and feeding behaviors.” From there, Duvall’s research took off. She proceeded to identify the exact receptor—Neuropeptide Y-like receptor 7 (NPYLR7)—activated by these drugs and modified the receptor’s gene in the mosquitoes to show, genetically, the importance of the receptor in regulating host-seeking and feeding behaviors. This important work of identifying the target for blocking mosquito biting was published in Cell in 2019.
Although “missing the FlyBase [a Drosophila genetic and molecular database] so much” and facing the challenge of having to start from scratch, Duvall says she came to enjoy building her own tools for mosquito research. She recalls the days when she was busy gluing together laser-cut plastic pieces for a mosquito behavior assay and first using the newly emerged editing tool CRISPR-Cas9 for mutant design.
Now in her own lab, while digging deeper into the three fundamental behaviors in mosquitoes—timing, feeding and mating—she is asking if this knowledge can be applied to tackle real-world problems.
Aedes aegypti mosquitoes, the species that Duvall mostly studies, were historically known as the yellow-fever mosquitoes. Nowadays, they also spread the pathogens that cause dengue, Zika and chikungunya. Therefore, finding an approach to control the feeding and mating behaviors of these dangerous animals will be of great benefit to humans. In mosquitoes, the male releases a signal to enforce paternity during mating with a female, preventing her from mating with a second male. In 2017, Duvall pulished a study reporting a peptide, HP-I, in males that can effectively change the mating behavior in females. Now, Duvall is trying to map out the reproductive circuity and understand how this cue changes female behavior. “The knowledge we learn here can be really important to inform the applications of these strategies,” says Duvall.
Taking advantage of this “monandry” phenomenon, the strategy of releasing sterile male mosqutioes has gained some success in vector control. Sterile males father no offspring, but prevent the female from accepting another, fertile mate. Previous work has shown that when multiple mosquito species cohabitate and reproductively interefere with each other, new mating strategies can evolve so that females mate with multiple males—which renders the sterile male technique ineffective. Duvall’s group is also working to understand if cohabitation history affects mosquito mating signals or circuitry.
LAURA DUVALL
Duvall is also working to identify new drugs that can either turn on or turn off the signaling pathways that control mosquito feeding and mating behaviors. “For example, it will be very useful if you can put some drugs in the environment to turn off the biting urge for a couple of days or get the female mosquitoes to reject all males.”
As a new investigator, Duvall is glad that the awards and grants she received can support piloting some risky studies such as the inter-species interaction. “Science is always intimidating and exciting for me,” says Duvall.
Laura Duvall, PhD
Assistant Professor, Department of Biological Sciences, Columbia University
WashU training: DBBS neuroscience PhD program, 2007-2012
Graduate advisor: Paul Taghert, PhD
Expertise: Neuropeptide regulation of behavior
Favorite St. Louis spot: City Museum
WashU highlight: Neuroscience student retreat