News
Advancing comparative and translational neuroimaging methodologies
Joonas Autio, PhD, an assistant professor of neuroscience and radiology, has over 15 years of experience in comparative magnetic resonance imaging.
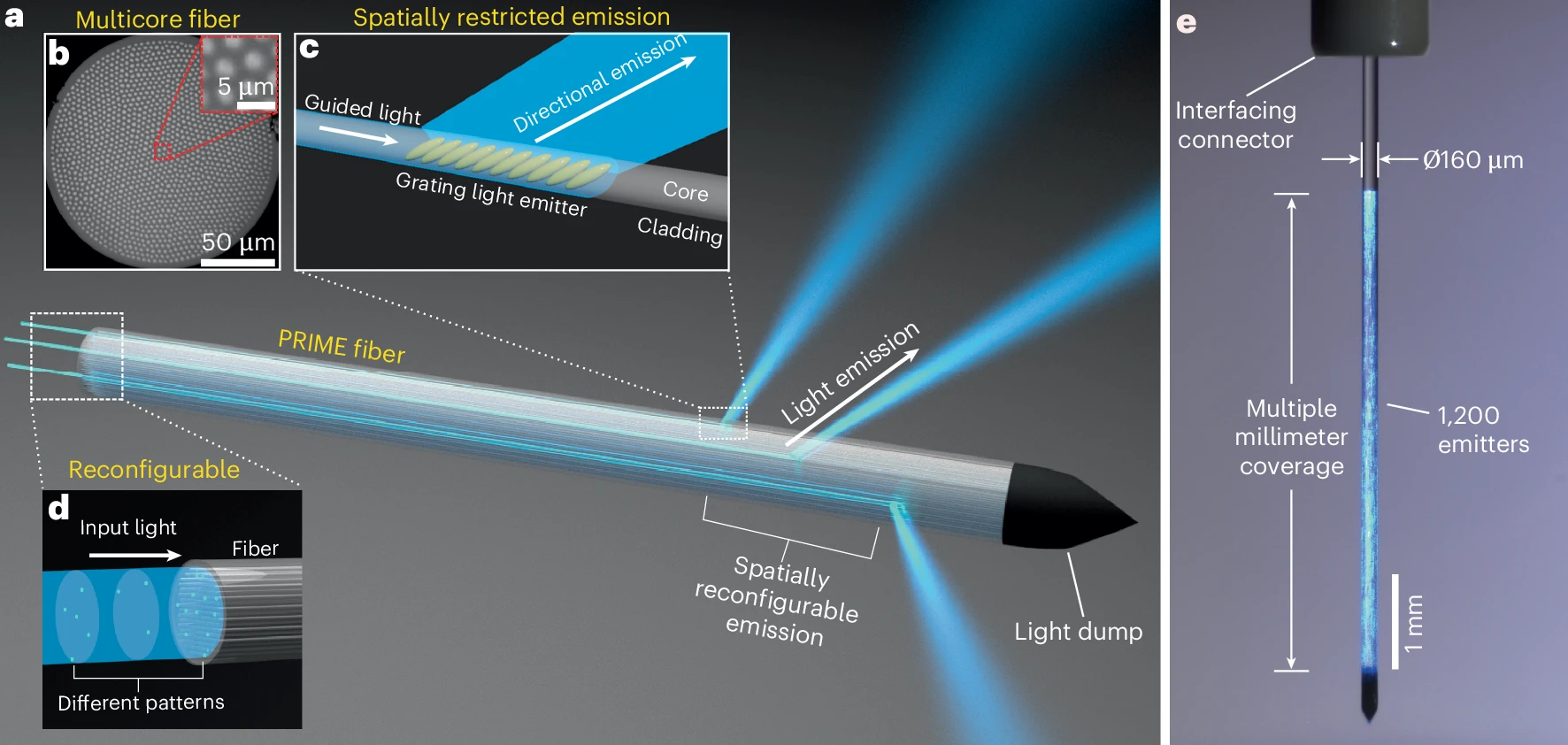
Prime time for fiber optics to take a deep dive into brain circuits (Links to an external site)
A group of researchers from the McKelvey School of Engineering and the Kepecs Lab has created a new kind of fiber-optic device to manipulate neural activity deep in the brain.
Watch the 2025 Distinguished Lecture
Watch the 2025 Department of Neuroscience Distinguished Lecture presented by Michael Rosbash, PhD, a 2017 Nobel Prize laureate in Physiology or Medicine.
Publications
Laser-engineered PRIME fiber for panoramic reconfigurable control of neural activity
Researchers from the McKelvey School of Engineering and the Kepecs Lab have a new paper in Nature Neuroscience describing their development of a panoramically reconfigurable illuminative (PRIME) that offers a powerful platform for optical control of neural circuits across the brain.
Events
-
4 Mar
-
4 Mar
-
6 Mar
-
11 Mar
Join our team of dedicated faculty, motivated trainees and passionate staff members.

Exceptional training
Providing foundational training, grounded in anatomy, at all levels
- Exciting, supportive environment
- Award-winning instructors
- Diverse mentorship opportunities
Our city will surprise you
St. Louis is an affordable, family- and foodie-friendly city with amazing green spaces and incredible cultural activities.