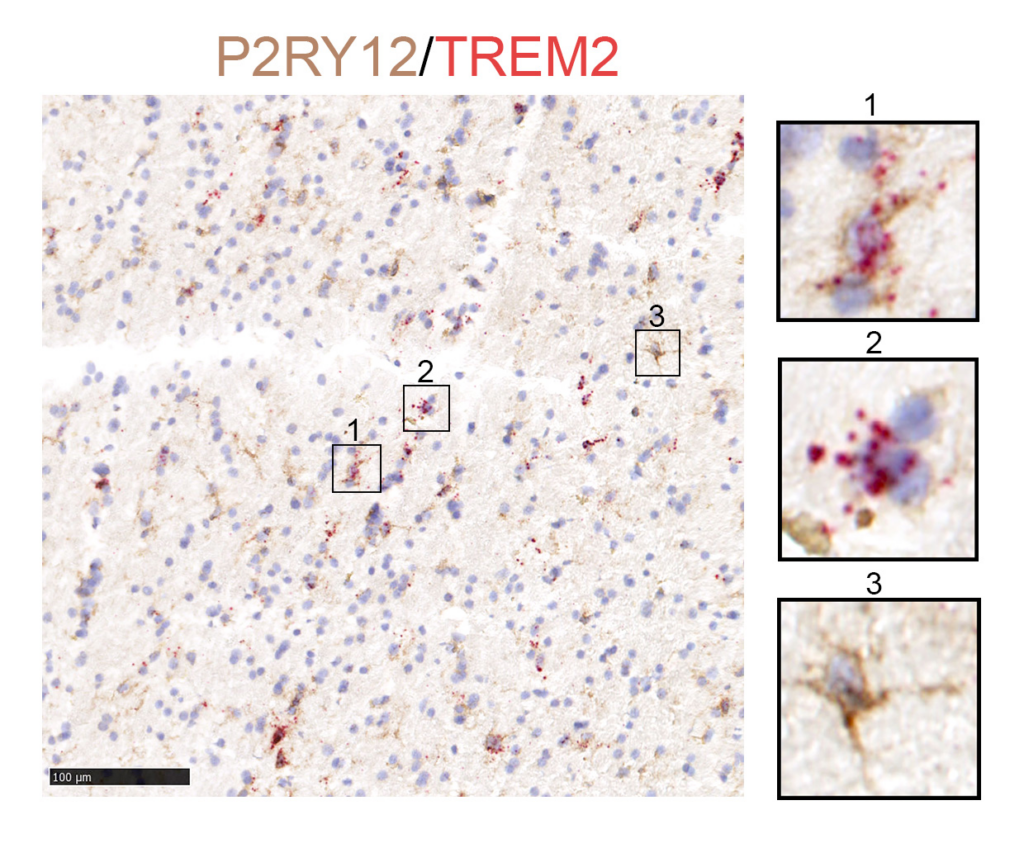
COURTESY GUOYAN ZHAO, PHD
Neurodegenerative disorders such as Alzheimer, Parkinson and Huntington disease each cause their own distinct set of symptoms, yet they share a common feature: abnormal protein aggregation. It’s been unknown if overlapping cellular pathways underly the damaging accumulation of proteins in these diseases. Mounting evidence demonstrates how glial cells such as astrocytes and microglia play a key part in neurodegenerative disease. However, most current knowledge is derived from in vitro systems or animal models, whose relevance to human disease remains under debate, and suspicion has been placed on glia—nonneuronal cells with various critical functions in nervous system health. Guoyan Zhao, PhD, Assistant Professor of Neuroscience, and colleagues at Washington University School of Medicine report in Nature Aging transcriptomic profiles of glia from human brain samples donated by individuals with neurodegenerative disease and healthy controls. Although they identified genetic activity in glia common to all individuals, the researchers also discovered disease-specific gene expression in microglia, known as the brain’s resident immune cells, and astrocytes, another type of glia, associated with neuropathology and neurodegeneration.
Prior studies of glial participation in neurodegeneration have relied upon laboratory studies of animals or cells. In this latest work, the authors collected brain samples from deceased donors and analyzed more than 30,000 single-nucleus transcriptomes. They compared these data to previously published transcriptomes of other brain regions from human donors.
The research team identified several astrocyte and microglia subpopulations common among all these samples, including from people with and without neurodegenerative disease. These samples also shared signs of genetic activity associated with microglia activation—an indication of neuroinflammation present even in healthy brain tissue. “This finding supports the idea that reactive microglia share a common response to central nervous system challenges,” said Zhao.
Differences also emerged between the groups. Astrocytes from individuals with Alzheimer and Parkinson disease had overlapping transcriptome features that were distinct from the transcriptomes of healthy controls, and these differentially expressed genetic pathways were linked with amyloid-ß pathology and neurodegeneration.
Together with our finding that astrocyte and microglia transcriptomic changes contribute to neurodegeneration and neuropathology in human brains, these findings provide guidance for future mechanistic study to understand the role of glia in disease pathogenesis using mouse models.
Guoyan Zhao, PhD
Microglia from individuals with neurodegenerative disease revealed disease-specific transcriptome profiles, and microglia from different brain regions were affected differently. This might explain why certain brain regions are more vulnerable to pathological protein aggregation than others in these diseases. Among samples from affected individuals, transcriptome features associated with microglia activation were tied to amyloid-ß pathology, tauopathy and neuronal death.
The results contribute to delineating the roles of glia in neurodegenerative diseases. Given that the data represent one point in time, it’s impossible to determine if the transcriptional alterations are a cause or consequence of the disorder, but they narrow down the pool of candidate genes from which to identify causal actors and potentially intervene.
“Our work is the first study to demonstrate that astrocyte and microglia subpopulations are shared across multiple brain regions and conserved between human and mice,” said Zhao. “Together with our finding that astrocyte and microglia transcriptomic changes contribute to neurodegeneration and neuropathology in human brains, these findings provide guidance for future mechanistic study to understand the role of glia in disease pathogenesis using mouse models.”
The project was a collaboration among researchers from the labs of Jinbin Xu, Associate Professor of Radiology, Tristan Qingyun Li, PhD, and Jason Yi, PhD, Assistant Professors of Neuroscience, Randall Bateman, MD, the Charles F. and Joanne Knight Distinguished Professor of Neurology, Tammie L.S. Benzinger, Professor of Radiology, Ting Wang, PhD, Professor of Genetics, Professor of Computer Science and Engineering, and the Sanford and Karen Loewentheil Distinguished Professor of Medicine, Joel Perlmutter, MD, the Elliot H. Stein Family Professor of Neurology, and John Morris, MD, the H.A. & D. Hacker Friedman Distinguished Profressor of Neurology. The research was supported by the McDonnell Genome Institute at Washington University, the National Institutes of Health, the Philip and Sima Needleman Student Fellowship in Regenerative Medicine and the Philippine Department of Science and Technology.