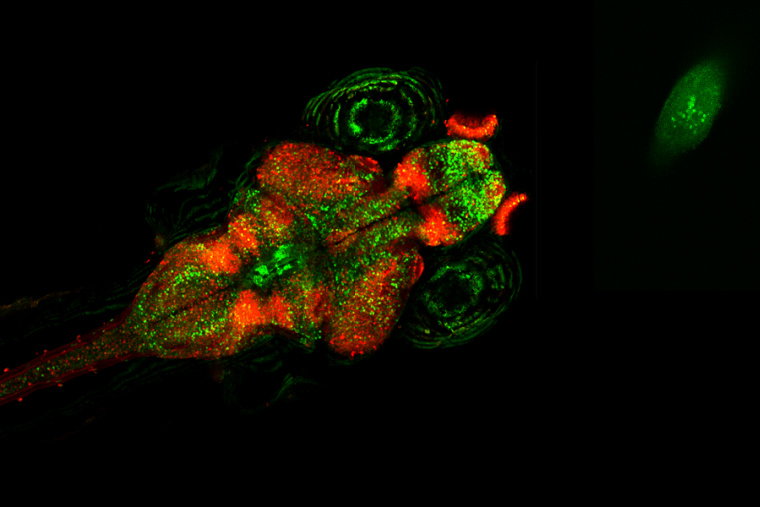
Fragile X Syndrome is the most common inherited form of autism, caused by variants in the FMR1 gene. Scientists have developed animal models of the disorder to better understand the consequences of the genetic mutation—and to see if they can intervene. In a recent study in The Journal of Neuroscience, Geoffrey Goodhill, PhD, Professor of Neuroscience and Developmental Biology at Washington University School of Medicine, and his team describe the utility of zebrafish larvae in recapitulating features of Fragile X. Fish with the genetic variant displayed alterations in the shape of their face, their hunting and social behaviors, and in neural activity.
For Goodhill, the most striking result was the fish’s aversion to a stimulating environment, a behavior also seen in individuals with Fragile X. “I’m not aware that anything like this has been seen in a zebrafish autism model before,” said Goodhill. Rearing the fish in a less stimulating environment corrected altered neural activity in the visual center of the brain caused by the fmr1 mutation. “This provides confirmation that sensory defensiveness in ASDs [autism spectrum disorders] applies across species, and suggests intriguing avenues for future work using zebrafish models,” the authors, led by postdoctoral researcher Shuyu Iris Zhu, PhD, write in their paper.
Fragile X affects roughly 1 in every 7,000 males and 1 in every 11,000 females. In addition to autism, individuals also typically have intellectual disability and a long, narrow face. Scientists had previously developed models of Fragile X Syndrome using zebrafish with mutations in fmr1. Prior work focused on the sensory and behavioral changes in fish with fmr1 variants, and Goodhill’s group wanted to further examine how visually directed behaviors and their correlated neural coding are affected.
Using fish lacking fmr1 they observed features analogous to those individuals with Fragile X, including altered craniofacial shape and social behaviors. When pairs of fish were placed in a behavioral assay—either two control fish or one control and one mutant—fish with the genetic variant tended to spend more time close to the other fish, but lagged in responding to that other fish’s movements.
These results suggest interesting new directions for using zebrafish as a model to study the development of brain and behavior in ASDs [autism spectrum disorders], and how the impact of ASDs could potentially be reduced.
Zhu et al., Journal of Neuroscience
Given that sensory deficits are common in Fragile X, the researchers sought to measure how other visually guided behaviors are affected. In one set of experiments, they tracked how well the zebrafish captured their paramecium prey. Compared to control fish, the Fragile X models were less successful hunters and video analysis of their movements and eye gaze identified alterations in the sequence of swimming moves used during hunting. They then examined cellular activity in the optic tectum of the brain, a visual-processing region crucial for hunting, while presenting the fish with visual stimuli. Not only did individual neurons respond differently to visual cues, but neural assemblies in the tectum—groups of coordinated cells—included greater numbers of neurons and appeared to be more excitable, perhaps explaining why hunting behavior was suboptimal.
In their examination of how zebrafish lacking fmr1 respond to different visual environments, they placed fish in chambers with one half of the bottom presenting an image of gravel and the other half featureless. While control fish showed no preference for either side, the Fragile X model preferred swimming above the plain side of the chamber. Turning again to the tectum, the researchers found that mutant fish raised in a tank with a gravel bottom had more excitable neural assemblies than did those raised in a plain tank, and the plain-tank fish had neural activity similar to control fish.
These results indicate that a less-stimulating environment might correct alterations in the tectum due to the absence of fmr1, and Goodhill said further investigation of the effects of reduced stimulation on Fragile X models is warranted.
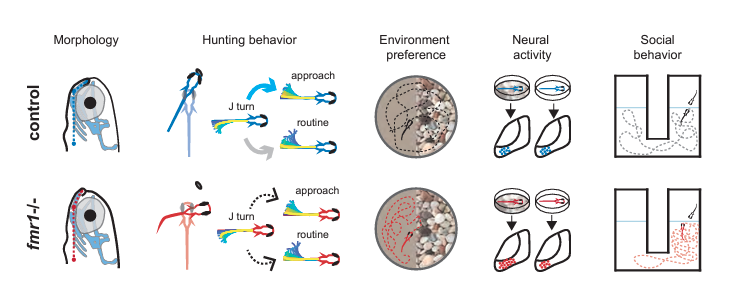
ZHU ET AL., J NEUROSCI, 2023
His team’s next step is to expand their analysis of the brain from approximately 200 neurons in the tectum as they did in this study to the entire zebrafish brain, around one hundred thousand neurons. This would provide a more comprehensive view of the effects of fmr1’s loss. They also plan to expand their approach to modeling Rett Syndrome, another form of autism caused by variants in a single gene, MECP2. In their paper the authors write, “These results suggest interesting new directions for using zebrafish as a model to study the development of brain and behavior in ASDs [autism spectrum disorders], and how the impact of ASDs could potentially be reduced.”