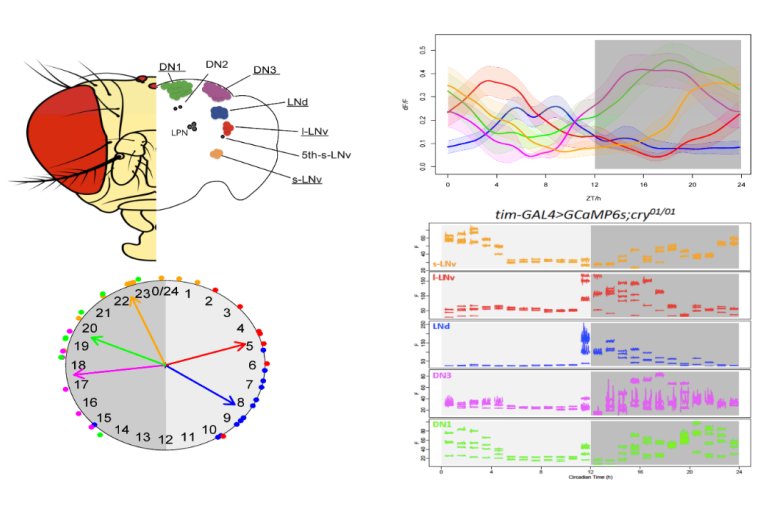
Across the circadian cycle, tides of molecular activity roll in and out within pacemaker neurons—genes turn on and off, electrical activity surges and wanes, calcium levels rise and fall. These fluctuations are observed in organisms as far-flung on the tree of life as bacteria and humans. For the most part, scientists have tracked molecular circadian rhythms at the resolution of minutes to hours. Recent work by a group in the Department of Neuroscience at Washington University School of Medicine reports on daily regulation of calcium activity across the 150-neuron network of circadian pacemakers within the fruit fly brain, at high frequencies over the course of 24 hours. Calcium signaling is widely appreciated in circadian physiology as an important component of both input to the clock, as well as output from it.

The newly published work was part of the PhD thesis studies of Xitong Liang, co-mentored by Paul Taghert, PhD, Professor of Neuroscience, and Tim Holy, PhD, the Alan A. & Edith L. Wolff Professor of Neuroscience at the School of Medicine. The group identified two layers of circadian rhythms in calcium levels, one slow and one fast, that are linked to one another: as the tide of calcium levels peaks in the slow cycle, so does the size of the waves in the fast rhythms. The results, published in PNAS, illuminate a coordinated mechanism of regulating pacemaker cell function through different calcium-related cellular activities.
The study builds upon a discovery reported in Science by the Taghert and Holy Labs, again led by Xitong Liang in 2016, that intracellular calcium cycles daily in Drosophila pacemaker neurons. Unexpectedly, in that prior study, the phase of the calcium cycling differed in different pacemakers, even though they all tell the same molecular time according to their intracellular molecular clock. Those original calcium measurements were taken every 10 minutes over a 24-hour period.
“Our assumption from the earlier work was that the slow rhythms we discovered reflected periods of neuronal electrical activity. In other words, daily calcium fluxes, as a proxy for neuronal activity, represent circadian output,” said Taghert. However, learning whether fast fluctuations in calcium (related to neuronal firing) might also exhibit circadian rhythms would require faster sampling rates, a feat not possible at the time.

The Holy lab, just down the hall from the Taghert Lab, was working on a solution. In 2019, Holy and a Cody Greer, another Neuroscience PhD student, reported the development of refinements to the original Objective Coupled Planar Illumination (OCPI) microscopy, which they termed OCPI-2. OCPI is a form light sheet fluorescence microscopy that can sample cellular activities in three dimensions at high speed—and the OCPI-2 refinements could support sampling rates high enough to capture the fast calcium fluctuations predicted by the earlier work.
Liang, Holy and Taghert took advantage of OCPI-2 to image calcium dynamics in the fruit fly brain over 24 hours at rates as fast as 5 Hz for periods of a minute every hour. This revealed the original slow and the predicted fast cycles, and their co-phasic relationship.

To determine the sources of these rhythms, they knocked-down various calcium channels from the cells using RNA-interference methods. They found disruption to the slow cycle when the IP3 receptor present on endoplasmic reticulum was knocked down, indicating that this flux is due mainly to changes in internal calcium stores. The fast cycle was interrupted when a T-type voltage-gated calcium channel was impaired, providing evidence that external calcium influx from electrical activity is responsible for the fast rhythms.
“Interestingly, knocking down levels of the IP3R also affected the fast rhythm, which may indicate a functional link whereby the slow wave underlies generation of the fast rhythm,” said Taghert. Altogether, the findings point to coupled rhythms generated by different cellular processes that regulate the function of pacemaker neurons. They provide a framework to extend the study of circadian pacemaker physiology. Future goals might include definition of the molecular pathways by which the clock regulates daily fluxes of calcium from internal stores, and the pathways providing linkage to daily regulation of channel activity in the plasma membrane.