A new study from Yao Chen’s lab reveals that some fluorescent-intensity–based sensors also show fluorescence lifetime responses, expanding observations of neuromodulator activity over time and distance.
Neuromodulators such as dopamine and acetylcholine are at the root of critical brain functions such as sleep, learning and mood. Tracking their dynamics provides insight into their roles, but conventional imaging techniques based on the intensity of a fluorescent reporter have been limited in how long they can monitor neuromodulator dynamics and over what distance in the brain. Researchers in the lab of Yao Chen, PhD, Assistant Professor of Neuroscience at Washington University, report that fluorescence lifetime imaging—a method that measures the duration a sensor is in an excited state—can pick up where intensity-based imaging falls short.

“With our method, now we can record both transient and chronic changes of neuromodulators simultaneously and distinguish their contributions,” says lead author Pingchuan Ma, a graduate student in the Chen Lab. “For example, we can now identify which types of the dynamical changes in neuromodulators are the main drivers for chronic disease pathogenesis, for responses to drug treatments, and for chronic biological processes such as development and aging.”
Ma, Chen and colleagues published their findings in Science Advances.
To fully understand how neuromodulators influence neural circuitry and function, it’s necessary to follow both their transient and sustained activities. Fluorescence intensity does not just change in response to neuromodulator concentrations, but also increases as sensor expression increases, and so cannot be used to make comparisons of neuromodulator concentrations across brain regions, across animals, or across chronic periods. Because fluorescence lifetime is indifferent to sensor expression levels and fluctuations in excitation light power, it provides consistency across time and distance that intensity-based reporters lack, yet its application for neuromodulator sensors was untested, and neither was it known how stable fluorescence lifetime is over long periods of time.
The Chen Lab screened several intensity-based neuromodulator sensors, all equipped with a single fluorophore, to see if they would show fluorescence lifetime change. To the authors’ surprise—given that very few single fluorophore–based molecular sensors are known to display fluorescence lifetime change—several neuromodulator reporters showed fluorescence lifetime responses.

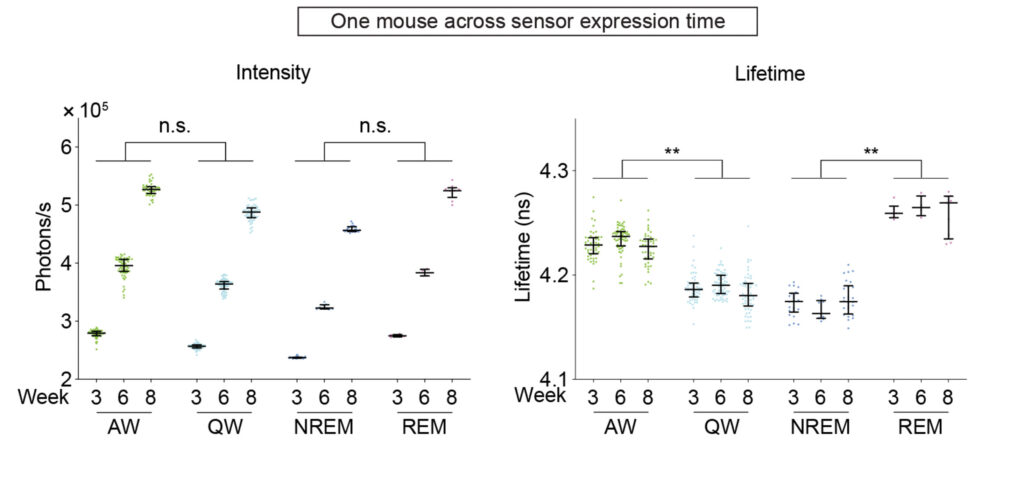
Because the acetylcholine sensor GRABACh3.0 showed the largest fluorescent lifetime change, the researchers put it through a battery of tests to assess its utility in fluorescence lifetime imaging and photometry. They found it to be a robust reporter, faithfully capturing acetylcholine dynamics across short and long time scales. When applied in vivo in mice, fluorescence lifetime of GRABACh3.0 accurately reported neuromodulator activity during distinct behaviors, namely, running and resting, and reliably tracked acetylcholine dynamics across sleep and wake cycles among different animals over the course of weeks.
“The results present opportunities to use fluorescence lifetime measurement of neuromodulator sensors to study the dynamics of these important molecules both at high spatial and temporal resolution, and compare both slow and fast dynamics across animals, brain regions, and chronic time scales,” said Chen.
“Our findings will also inspire the sensor developers to develop more fluorescence lifetime imaging–based neuromodulator sensors and empower scientists who are studying neuromodulators to explore the functions of neuromodulators with various types of dynamical changes,” added Ma.